Abiraterone acetate
Abiraterone acetate, inayouzwa chini ya jina la chapa Zytiga miongoni mwa zingine, ni dawa inayotumika kutibu saratani ya kibofu.[6] Hutumika hasa pamoja na kotikosteroidi kwa saratani ya tezi dume inayostahimili kuhasiwa (mCRPC) na saratani ya tezi dume yenye hatari kubwa ya kuhasiwa (mCSPC).[1] Inapaswa kutumiwa kufuatia kuondolewa kwa korodani au pamoja na analogi ya gonadotropini-itoayo homoni (GnRH).[1] Inachukuliwa kwa mdomo.[6]
| |
|---|---|

| |
| Jina la Utaratibu la (IUPAC) | |
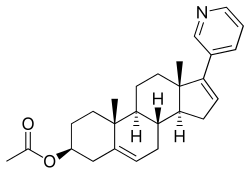
| [(3S,8R,9S,10R,13S,14S)-10,13-dimethyl-17-pyridin-3-yl-2,3,4,7,8,9,11,12,14,15-decahydro-1H-cyclopenta[a]phenanthren-3-yl] acetate | |
| Data ya kikliniki | |
| Majina ya kibiashara | Zytiga, Yonsa, mengineyo |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a611046 |
| Taarifa za leseni | EMA:[[[:Kigezo:EMA-EPAR]] Link], US Daily Med:link |
| Kategoria ya ujauzito | D(AU) ?(US) |
| Hali ya kisheria | Prescription Only (S4) (AU) ℞-only (CA) POM (UK) ℞-only (US) ℞ Prescription only |
| Njia mbalimbali za matumizi | Kwa mdomo (vidonge)[1][2] |
| Data ya utendakazi | |
| Uingiaji katika mzunguko wa mwili | Haijulikani, lakini inaweza kuwa 50% zaidi kwenye tumbo tupu[3] |
| Kufunga kwa protini | Abiraterone: ~99.8% (kwa albumin na Kigezo:Abbrlink)[3][1][4] |
| Kimetaboliki | Esterases, CYP3A4, SULT2A1[4] |
| Nusu uhai | Abiraterone: Masaa 12–24[1][3][2] |
| Utoaji wa uchafu | Kinyesi: 88%[1][4] Mkojo: 5%[1][4][2] |
| Vitambulisho | |
| Nambari ya ATC | ? |
| Visawe | CB-7630; JNJ-212082; 17-(3-Pyridinyl)androsta-5,16-dien-3β-ol acetate |
| Data ya kikemikali | |
| Fomyula | C26H33NO2 |
| |
| Data ya kimwili | |
| Kiwango cha kuyeyuka | 144–145 °C (291–293 °F) [5] |
| | |
Madhara yake ya kawaida ni pamoja na uchovu, kutapika, maumivu ya kichwa, maumivu ya viungo, shinikizo la damu, uvimbe, potasiamu katika damu, sukari ya juu ya damu, kuwaka moto, kuhara na kikohozi.[6][1] Madhara yake mengine makubwa yanaweza kujumuisha kushindwa kwa ini na upungufu wa adrenali.[1] Kwa wanaume ambao wenzi wao wanaweza kupata mimba, udhibiti wa kuzaliwa unapendekezwa.[1] Ikitolewa kama abiraterone acetate, inabadilishwa mwilini kuwa abiraterone.[1] Abiraterone acetate hufanya kazi kwa kukandamiza utengenezaji wa androjeni - haswa huzuia CYP17A1 - na hivyo kupunguza uzalishaji wa testosterone.[6] Kwa kufanya hivyo, huzuia athari za homoni hizi katika saratani ya kibofu.[6]
Abiraterone acetate ilielezewa katika mwaka wa 1995, na kuidhinishwa kwa ajili ya matumizi ya kimatibabu nchini Marekani na Ulaya katika mwaka wa 2011.[7][1] Iko kwenye Orodha ya Dawa Muhimu ya Shirika la Afya Ulimwenguni.[8] Nchini Uingereza, iligharimu NHS £2,700 kwa mwezi kufikia mwaka wa 2018.[9] Nchini Marekani, kiasi hiki kiligharimu $3,300 kufikia mwaka wa 2019.[10] Dawa hii inauzwa kote ulimwenguni.[11]
Marejeleo
hariri- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 "Zytiga- abiraterone acetate tablet, film coated". DailyMed. 13 Juni 2019. Ilihifadhiwa kwenye nyaraka kutoka chanzo mnamo 13 Novemba 2014. Iliwekwa mnamo 15 Novemba 2019.
{{cite web}}: CS1 maint: date auto-translated (link) - ↑ 2.0 2.1 2.2 "Yonsa- abiraterone acetate tablet". DailyMed. 5 Juni 2018. Ilihifadhiwa kwenye nyaraka kutoka chanzo mnamo 13 Agosti 2020. Iliwekwa mnamo 15 Novemba 2019.
{{cite web}}: CS1 maint: date auto-translated (link) - ↑ 3.0 3.1 3.2 Benoist GE, Hendriks RJ, Mulders PF, Gerritsen WR, Somford DM, Schalken JA, van Oort IM, Burger DM, van Erp NP (Novemba 2016). "Pharmacokinetic Aspects of the Two Novel Oral Drugs Used for Metastatic Castration-Resistant Prostate Cancer: Abiraterone Acetate and Enzalutamide". Clin Pharmacokinet. 55 (11): 1369–1380. doi:10.1007/s40262-016-0403-6. PMC 5069300. PMID 27106175.
{{cite journal}}: CS1 maint: date auto-translated (link) - ↑ 4.0 4.1 4.2 4.3 "Meeting Library - Meeting Library". meetinglibrary.asco.org. Ilihifadhiwa kwenye nyaraka kutoka chanzo mnamo 20 Septemba 2016. Iliwekwa mnamo 9 Septemba 2016.
{{cite web}}: CS1 maint: date auto-translated (link) - ↑ Potter, Gerard A.; Barrie, S. Elaine; Jarman, Michael; Rowlands, Martin G. (1995). "Novel Steroidal Inhibitors of Human Cytochrome P45017 alpha (17.alpha.-Hydroxylase-C17,20-lyase): Potential Agents for the Treatment of Prostatic Cancer". Journal of Medicinal Chemistry. 38 (13): 2463–2471. doi:10.1021/jm00013a022. ISSN 0022-2623. PMID 7608911.
- ↑ 6.0 6.1 6.2 6.3 6.4 "Abiraterone Acetate Monograph for Professionals". Drugs.com (kwa Kiingereza). Ilihifadhiwa kwenye nyaraka kutoka chanzo mnamo 6 Mei 2012. Iliwekwa mnamo 15 Novemba 2019.
{{cite web}}: CS1 maint: date auto-translated (link) - ↑ "Where did abiraterone come from?". Cancer Research UK. 2011-09-21. Ilihifadhiwa kwenye nyaraka kutoka chanzo mnamo 25 Septemba 2011. Iliwekwa mnamo 2011-09-28.
{{cite web}}: CS1 maint: date auto-translated (link) - ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ British national formulary : BNF 76 (tol. la 76). Pharmaceutical Press. 2018. uk. 921. ISBN 9780857113382.
- ↑ "Abiraterone Prices, Coupons & Patient Assistance Programs". Drugs.com (kwa Kiingereza). Ilihifadhiwa kwenye nyaraka kutoka chanzo mnamo 9 Oktoba 2019. Iliwekwa mnamo 15 Novemba 2019.
{{cite web}}: CS1 maint: date auto-translated (link) - ↑ "Abiraterone". Drugs.com. Ilihifadhiwa kwenye nyaraka kutoka chanzo mnamo 30 Novemba 2014. Iliwekwa mnamo 14 Aprili 2018.
{{cite web}}: CS1 maint: date auto-translated (link)